
Sub-visible Particle Analysis
What are particles?
Particles and particulate matter are everywhere: They are “the tiny bits of matter that make up the universe”. In fact, even if we do not see them, particles of all size ranges are an essential part of many highly relevant domains.They influence climate, water quality, health and viability of organisms, and material structures. Further, their detection and characterization are essential in diverse fields such as electronics, pollution control, cosmetics and pharmaceuticals.
Definition: What is Particulate Matter?
“mobile undissolved particles, other than gas bubbles, unintentionally present in the solutions.” (United States Pharmacopeia)
Particles in Medicines
In the pharmaceutical industry, particulate matter is an obligatory critical quality attribute (CQA) for parenterally administered medicines, i.e. medicines that are delivered by injection or infusion. Particles may impact patient safety and have been suspected in relation to vascular occlusions and immune responses. In addition, the occurrence of particles can be a sign of product instability and issues related to product quality.

Assessing particulates is key at all stages of a pharmaceutical product – during development, manufacturing, testing and stability.
Dr. Hanns-Christian Mahler
Not all particles are the same
Particles in medicines differ, for example in
- Type (chemical composition) and source, e.g., cellulose fibers, glass, rubber, plastic, metal, barium sulfate, protein, silicone droplets
- Morphology
- Size: particles can be visible or sub-visible (non-visible)
The USP has suggested different particle categories:
- extrinsic particles – unexpected foreign material such as cellulose;
- intrinsic particles – resulting from addition or by insucient cleaning during manufacturing, such as tank metals or gaskets, lubricants, lling hardware, or resulting from instability;
- inherent particles, which originate from the active pharmaceutical ingredient (the protein) or the drug delivery vehicle (formulation compounds).

There are no particle-free parenteral products.
Dr. Tobias Werk
Regulations related to Particulates
The United States Pharmacopeia (USP), the European Pharmacopoeia (EP) and the Japanese Pharmacopoeia (JP) have introduced stringent requirements over the past few years regarding visible and sub-visible particles.
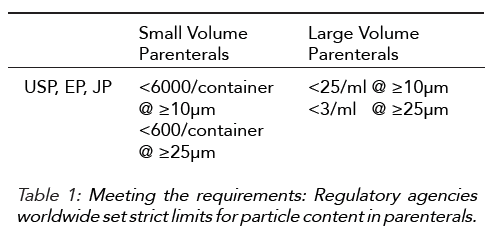
Generally speaking, there is growing concern about the presence of particles in parenterals, a need for effective particle control, as well as an ambition for industry requiring to be aiming at “zero particles” medicines. Visible particles are detectable by eye under defined conditions, hence typically 100um and larger. Parenteral preparations should be essentially free of visible particles. Sub-visible particles must be quantified and sized in buckets of ≥10µm and ≥25 µm. Parenteral preparations should comply with limits provided by the Pharmacopoeias or as approved for the particular product.
How can scientists count and size sub-visible particles?
The compendia have suggested two methods for the quantification of sub-visible particles:
- Microscopy
- Light Obscuration
In addition, USP has mentioned flow imaging as an optional method; however, there is no reference related to acceptance criteria

Whitepaper: Sub-visible Particle Testing
An overview of current analytical techniques and an outlook on innovation
Light obscuration: a standard method stuck in the past?
The preferred method for measuring sub-visible particles in compliance to compendias is light obscuration. Light obscuration is based on the physical principle of light blocking: When a particle goes through a light beam, it blocks the light and generates a “shadow”. In particle counters, the light source is a laser diode, and the “shadow” is detected by an optical sensor which is calibrated using particles of certified sizes and transferred into a particle size. As a measurement method, light obscuration offers a mature and robust platform for routine particle characterization since it quickly detects individual particles and provides highly accurate counts and sizes of sub-visible particles between 1 to around 150 µm in size.

Light obscuration also has some limitations. These include:
- Loss of the sample: The method is destructive, and samples cannot be reused.
- Labor-intensive and expensive procedure
- Need for high sample volumes (25ml/test)
- Limited workflow: only one measurement at a time
- Lower sensitivity in detecting some types of translucent, proteinaceous particles, resulting in lower counts
- Limited Information: only particle size and quantity
- Lower accuracy of analytical results when the difference is small between the refractive index of the particles and that of the medium
- Strong dependence of sizing accuracy on the properties of the reference material used for calibration and on the properties of the sensor
- Sensitivity to air bubbles and to some degassing procedures such as sonication since these can change sample properties
- Inability to detect particles smaller than 1µm
Other methods such as Flow Imaging, Background Membrane Imaging (BMI), or methods based on counting other physical principles have been assessed to overcome the limitations of light obscuration. However, these methods are not universally compendial; further, they are destructive, create difficult-to-interpret data and cannot be directly comparable with data from light obscuration.
Bionter’s solution: the EVE light obscuration particle counter
Bionter’s team has developed a new approach to light obscuration: the EVE Particle Counter. This revolutionary technological improvement will allow biopharmaceutical companies to greatly increase their workflow efficiency and will result in significant cost and time savings. This technology is:
- non-destructive
- fully automated
- compliant to Pharmacopoeias
- provides data integrity; FDA 21 CFR part 11 compliant