

EVE Particle Counter
EU Launch
CE certified since May 2022
US Launch
UL certification ready in Q4 2022
The EVE Particle Counter:
Operates non-destructively and preserves the test sample

Requires a smaller sample volume (0.1ml tare volume and zero pre-run needed)

Drives efficiency and saves money with smart workflow automation
Minimizes human interaction

Can easily replace existing light obscuration systems since it is compliant with current Pharmacopeia guidelines (for the US, EU and Japan)

Improves reliability, data integrity and, in turn, patient safety
Reliably detects up to 120k particles/ml before coincidence counting

Provides accurate measurements in a viscosity range of 1-150cp
A new approach in particle counting
The range of applications for sub-visible particle testing is wide: From stability testing to drug formulation and process development to in-process control testing, this test is used in many different steps of the biopharmaceutical workflow. Ultimately, the aim is for parenteral solutions to be virtually free of subvisible particles, which are those with a size between 100 nm and 100 µm. Otherwise, patient safety may be at stake. In addition, parenterals need to meet the criteria set by regulatory authorities, which define strict limit values for sub-visible particles
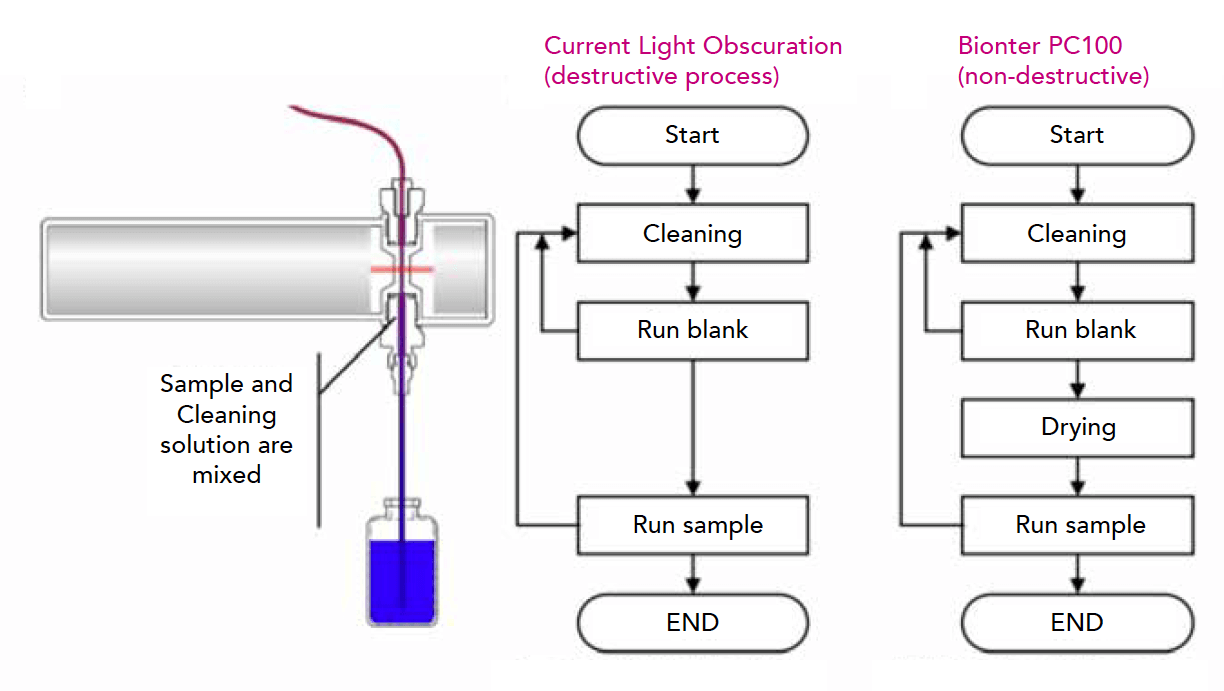
Current light obscuration technology: a compendial method needs to be revised
Given the crucial importance of accurate quantification and sizing of sub-visible particles in drug and process development, scientists would greatly benefit from a testing method which would allow them to maximize efficiency and minimize costs. To achieve these targets, two major challenges of the existing, compendial light obscuration systems need to be mastered: lack of automation and destruction of the sample.
In fact, minimal human interaction for sample preparation and handling reduces the chance of contamination and, thus, the percentage of false positive test results. The automation of this testing process would also increase process efficiency and reliability, which would ultimately result in increased safety for the patients. Moreover, non-destructive analysis methods would allow scientists to first assess the particle load using a compendial method and subsequently use orthogonal methods on the same sample for any follow-up investigations. A non-destructive technology would therefore avoid the loss of a considerable sample volume for testing purposes, which is especially expensive in the biotechnology sector. As an example, the cost of a single sample that is consumed typically ranges between $300 to more than $1,000.
The Bionter approach: changing old into new
In order to overcome these challenges, Bionter focused on developing a new, non-destructive, automated light obscuration approach. Unlike other instruments on the market, the EVE Particle Counter does not consume or destroy the sample during testing, allowing pharmaceutical companies to repeat testing on the same sample. This sample preservation results in significant financial and time savings during the development phase of new therapeutics.
The first step in the development of EVE was the identification of the reason for the destructive nature of the existing particle counters. The destructiveness was due to the mixing of the sample with the solutions used for cleaning and blank measurement. As the sample was no longer intact but ‘contaminated’ with the blank liquid, it was not possible to reuse it for further analyses. Therefore, we integrated an additional drying step after cleaning to avoid the contamination of the sample and to ensure a clean fluid path. EVE dries the fluid path to less than ⅕ of a single drop. Cleanliness is confirmed by using conductivity as an indicator.

In any business setting, a careful observer tries to find equipment and processes that can be improved. This was and remains exactly my goal: helping other scientists develop medicines faster and better.
Dr. Tobias Werk
Bionter’s EVE Particle Counter: a major step toward a fully automated workflow
Bionter’s EVE meets another challenge: The device is fully automated and runs unattended. This allows companies to make labor- and time-intensive tests much more efficient, increasing the level of automation and optimizing the analytical workflow. In fact, the introduction of EVE is a major step towards a lean analytical workstream. The light obscuration instrument consists of racks for primary packaging, a conveyor belt that transports the samples to an analysis area, bottles for cleaning media and waste, a fluidic system including the particle sensor, as well as single-use consumables that allow an efficient process. Users just need to load the samples onto the conveyor belt, refill the cleaning medium when necessary, empty the waste or refill the stack of single-use consumables.
Unlike other systems, the Bionter particle counter uses pressurized filtered air, rather than a syringe pump, to move the liquid sample. Further, it automatically adapts the pressure to the viscosity of each individual sample, ensuring an accurate and precise flow. With minimal human interaction, there is a reduced risk of operating errors and of sample contamination, as well as an increase in the reproducibility of the test results.
In addition, this revolutionary analytical sub-visible testing device is fully compliant with compendial requirements and can be used for development and quality control of both drug substances and drug products across the whole pharmaceutical product lifecycle.


The EVE Particle Counter
Performance
By loading the video, you agree to Vimeo's privacy policy.
Learn more
Working Principle

By loading the video, you agree to Vimeo's privacy policy.
Learn more

Contact us for more details
…and find out how our particle counter helps your lab to achieve a smart automation workflow.